UC Davis scientists contributed to the creation of a hybrid of silicon nanocircuits and biological components that mimics some of the processes that control the passage of molecules into and out of cells.
The lipid-coated nanocircuits could lead to the development of new classes of biosensing tools and biological applications, such as comprehensive blood-chemistry tests that fit on the point of a needle or screening tools for the development of new drugs.
“This is an example of a marriage between integrated circuit technology and biotechnology,” said Pieter Stroeve, a professor of chemical engineering and materials science at UC Davis and one of three lead scientists on the project. “The technology of both can be mass produced, so in theory, their integration can also be mass produced.”
The Proceedings of the National Academy of Sciences’ Aug. 18 edition carries a paper describing the research.
While modern communications devices rely on electric fields and currents to carry the flow of information, biological systems are much more complex. Their use of membrane receptors, channels and pumps to control chemical signals within and between cells is unmatched by even the most powerful computers. Because these complex operations have the potential for enhancing technological systems, scientists have been trying to develop techniques to blend the systems together.
An added advantage of such a fusion is its potential for miniaturization. “If you want to go to the nanoscale of circuit technology, some of the components need to be so small that they are either very difficult to produce, or they may fail,” Stroeve said. “On the other hand, many biological components have the tendency to self-assemble. And that’s what we built our work on.”
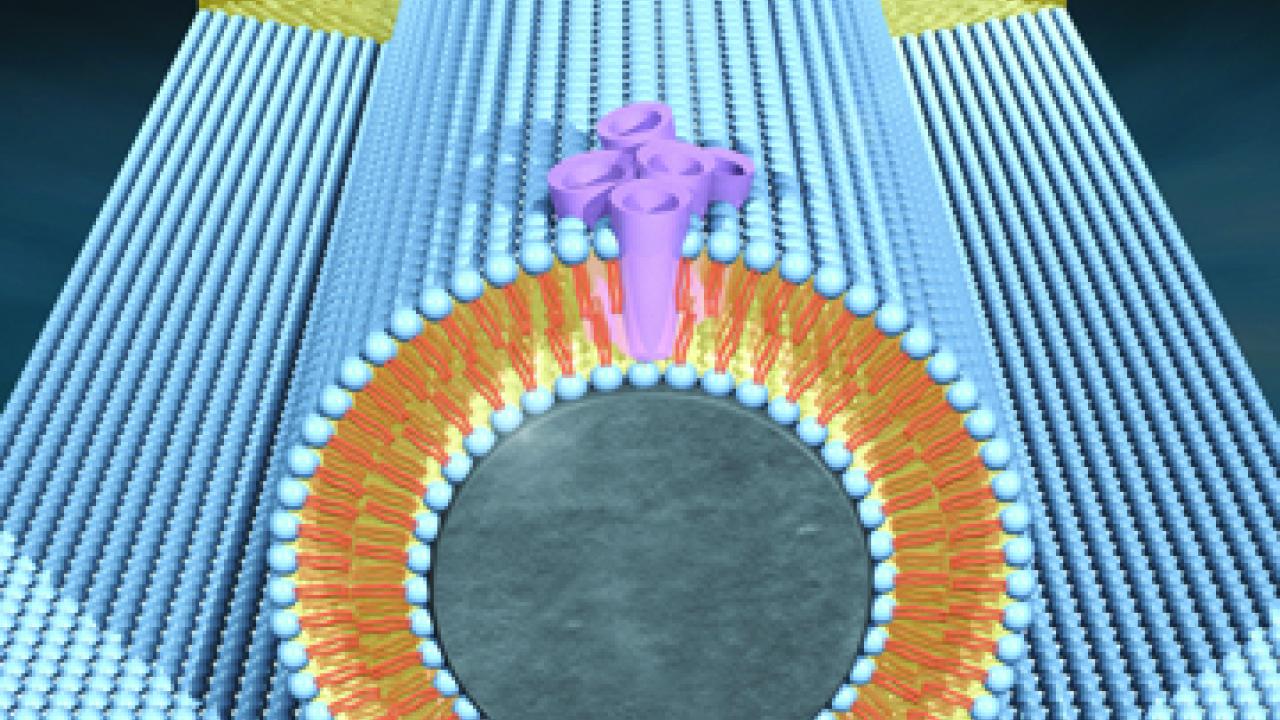
To create their hybrid system, Stroeve and lead scientist Aleksandr Noy, a chemist at Lawrence Livermore National Laboratory, and Costas Grigoropoulos, a professor of mechanical engineering at UC Berkeley, turned to lipid bilayer membranes: the thin films that surround cells, acting as gatekeepers for molecules passing into and out of the cell.
Using a technique they had previously developed, the team immersed nano-scale transistors made of silicon wire into a suspension of lipid molecules in water. Attracted to the negatively charged nanowire surfaces, the lipids accumulated onto them, forming a double layer that served to insulate the wires’ electrical properties as well as shield them from the surrounding water.
The team fused proteins from bacteria called Gramicidin A and Alemethicin into the lipid bilayers. In living cells these proteins serve as channels for molecules crossing the membrane.
By creating a voltage difference across the membrane, the researchers found that they could open and close the protein channels.
“This is much the same thing that happens in a cell,” Stroeve explained. “Now that we can open and close these channels, we can, in effect, regulate our system’s ability to sense chemicals in its environment.”
The two lead authors—Julio Martinez and Nipun Misra—
were graduate students when they did the work, Martinez at UC Davis, with Stroeve; and Misra at UC Berkeley. Other contributors: Shie-Chieh Huang at UCLA and Yinmin Wang at Lawrence Livermore National Laboratory.
Media Resources
Dave Jones, Dateline, 530-752-6556, dljones@ucdavis.edu